The Federal Circuit has held that the claims of a “SNALP” patent were inherently anticipated by prior art.
In Arbutus Biopharma Corp. v. ModernaTX, Inc., Arbutus appealed a decision in an inter partes proceeding by the Patent Trial and Appeal Board (PTAB) that found claims 1-22 of its U.S. Patent No. 9,404,127 invalid as anticipated.
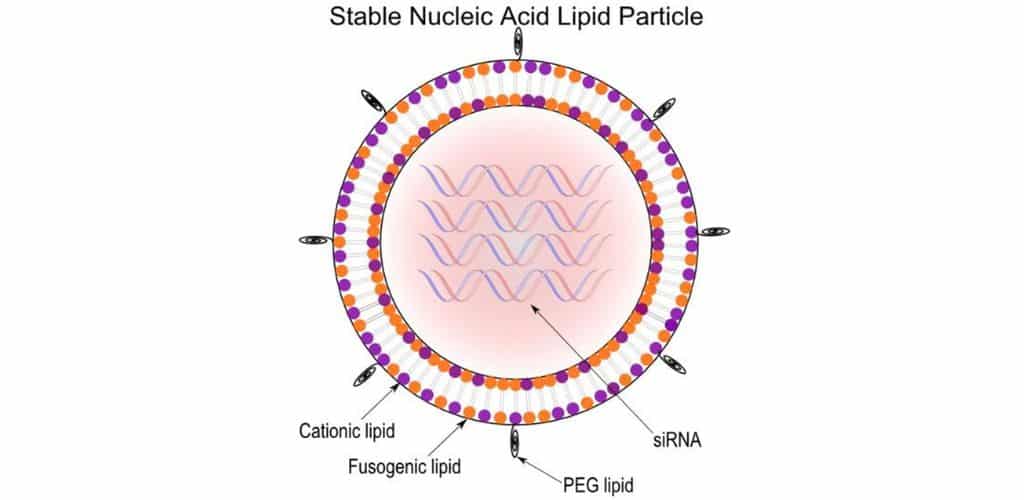
The patent is directed to an invention that provides stable nucleic acidlipid particles (“SNALP”) that have a non-lamellar structure and “comprise a nucleic acid . . . methods of making the SNALP, and methods of delivering and/or administering the SNALP.” The three-dimensional structure of SNALP is a physical property that has one of two morphologies: lamellar or nonlamellar.
A lamellar morphology is one in which sheets of lipid bilayers are arranged in layers, and a non-lamellar form refers to a non-bilayer morphology of the particles, such as in an inverse hexagonal structure.
The patent states that its purpose is to allow for more efficient methods and compositions for introducing nucleic acids into cells and methods of downregulating gene expression.
The physical property or morphology of the particles depends on two factors: (1) the lipids used for making the formulations and (2) the process used to form the particles.
Independent claim 1 claims:
- A composition comprising: a plurality of nucleic acid-lipid particles, wherein each particle in the plurality of particles comprises:
(a) a nucleic acid;
(b) a cationic lipid;
(c) a non-cationic lipid; and
(d) a conjugated lipid that inhibits aggregation of particles, wherein at least about 95% of the particles in the plurality of particles have a non-lamellar morphology. [Morphology Limitation]
Moderna filed a petition for inter partes review (IPR) challenging claims 1– 22 of the patent, arguing that U.S. Patent No. 8,058,069 anticipated every claim of the ‘127 patent.
The main question before the PTAB was whether claim 1(d) of the ’127 patent — wherein at least about 95% of the particles in the plurality of particles have a non-lamellar morphology (the “Morphology Limitation”) — is inherently disclosed in the ’069 patent.
Moderna argued that the Morphology Limitation, although not expressly mentioned in the prior art, is an “inherent natural property” resulting from the lipid composition of the formulation and formation process.
Arbutus countered that there was no presumption of inherency and no evidence that the ’069 patent and its formulations would necessarily have the same morphology as disclosed by the ’127 patent.
The Federal Circuit noted that
A claim is anticipated if each and every element as set forth in the claim is found, either expressly or inherently, in a single prior art reference. … A finding of anticipation “does not require the actual creation or reduction to practice of the prior art subject matter; anticipation requires only an enabling disclosure.”
Also,
A limitation is inherent if it is the “natural result flowing from” the prior art’s explicit disclosure. … A patent “can be invalid based on inherency when the patent itself makes clear that a limitation is ‘not an additional requirement imposed by the claims . . . but rather a property necessarily present’.”
Thus,
inherent anticipation requires “merely that the disclosure of the prior art is sufficient to show that the natural result flowing from the operation as taught in the prior art would result in the claimed product.”
The court noted that there was no dispute that the ’069 patent doesn’t explicitly teach the Morphology Limitation. However, the ’127 and ’069 patents disclose the same formulations with “almost identical wording,” and both patents disclose identical lipid compositions.
Thus, found the court, substantial evidence supports the PTAB’s finding that the formulations “are the same or essentially the same” across the patents.
Just like the haiku above, we like to keep our posts short and sweet. Hopefully, you found this bite-sized information helpful. If you would like more information, please do not hesitate to contact us here.